
HL Paper 2
Ethanedioic acid is a diprotic acid. A student determined the value of x in the formula of hydrated ethanedioic acid, \({\text{HOOC–COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\), by titrating a known mass of the acid with a 0.100 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of NaOH(aq).
0.795 g of ethanedioic acid was dissolved in distilled water and made up to a total volume of 250 cm3 in a volumetric flask.
\({\text{25 c}}{{\text{m}}^{\text{3}}}\) of this ethanedioic acid solution was pipetted into a flask and titrated against aqueous sodium hydroxide using phenolphthalein as an indicator.
The titration was then repeated twice to obtain the results below.

Calculate the average volume of NaOH added, in \({\text{c}}{{\text{m}}^{\text{3}}}\), in titrations 2 and 3, and then calculate the amount, in mol, of NaOH added.
The equation for the reaction taking place in the titration is:
\({\text{HOOC–COOH(aq)}} + {\text{2NaOH(aq)}} \to {\text{NaOOC–COONa(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Determine the amount, in mol, of ethanedioic acid that reacts with the average
volume of NaOH(aq).
Determine the amount, in mol, of ethanedioic acid present in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the original solution.
Determine the molar mass of hydrated ethanedioic acid.
Determine the value of x in the formula \({\text{HOOC–COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\).
Identify the strongest intermolecular force in solid ethanedioic acid.
Deduce the Lewis (electron dot) structure of ethanedioic acid, \({\text{HOOC–COOH}}\).
Predict and explain the difference in carbon-oxygen bond lengths in ethanedioic acid and its conjugate base, \(^ - {\text{OOC–CO}}{{\text{O}}^ - }\).
The vapour pressure of water changes with temperature according to the graph below.
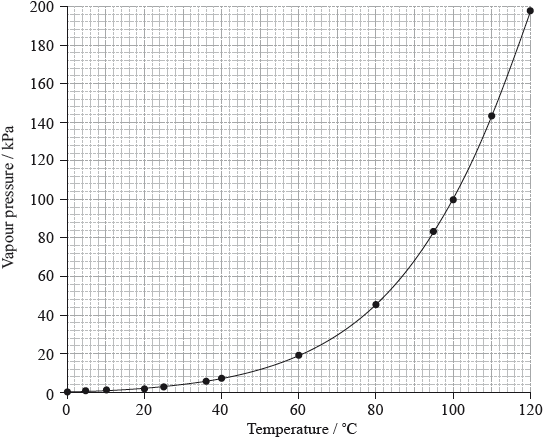
A liquid boils when its vapour pressure equals atmospheric pressure. Determine the boiling point of water on a mountaintop on a day when the atmospheric pressure is 60.0 kPa.
Sketch another curve on the axes above to show how the vapour pressure of a liquid that has weaker intermolecular forces than water, such as bromine, changes with temperature.
(i) A sample of liquid bromine was left in a closed conical (Erlenmeyer) flask at 298 K and allowed to reach a state of equilibrium. State an observation that indicates that equilibrium was reached.
(ii) The temperature of the closed flask was increased and the system was allowed to reach a new equilibrium. Compare the equilibrium formed at the new temperature with the equilibrium at the original temperature on the molecular level.
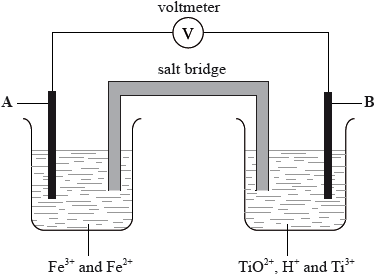
In acidic solution, ions containing titanium can react according to the half-equation below.
\({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\) \({E^\Theta } = - 0.06{\text{ V}}\)
In the diagram below, A and B are inert electrodes and, in the aqueous solutions, all ions have a concentration of \({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
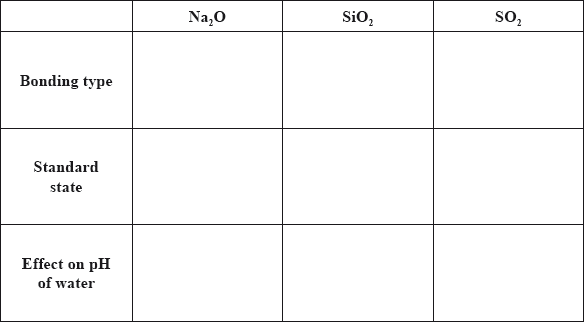
Sodium, silicon and sulfur are elements in period 3 of the periodic table that all form oxides.
Although carbon and silicon both belong to group 4 of the periodic table, carbon dioxide and silicon dioxide are different in many ways.
Define the term standard electrode potential, \({E^\Theta }\).
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
In the two experiments below, predict whether a reaction would occur and deduce an equation for any reaction that takes place. Refer to Table 14 of the Data Booklet if necessary.
KI(aq) is added to a solution containing \({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}}\) ions:
Zn (s) is added to a solution containing \({\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}}\) and \({{\text{H}}^ + }{\text{(aq)}}\) ions:
Using Table 14 of the Data Booklet, state the balanced half-equation for the reaction that occurs at electrode A and whether it involves oxidation or reduction.
Calculate the cell potential in V.
On the diagram above label with an arrow
• the direction of electron flow in the wire
• the direction in which the positive ions flow in the salt bridge.
Compare the properties of the three oxides by completing the table below.
Sulfur dioxide is a significant contributor to acid deposition. Identify a major, man-made source of this pollutant.
As well as the oxide above, sodium forms a peroxide that contains the peroxide ion, \({\text{O}}_2^{2 - }\). Draw the Lewis (electron dot) structure of the peroxide ion.
Describe the differences in the hybridization of these group 4 elements and the precise nature of the bonds that they form with the oxygen atoms.
Xenon, although a noble gas, forms an oxide, \({\text{Xe}}{{\text{O}}_{\text{2}}}\), that has a structure related to that of \({\text{Si}}{{\text{O}}_{\text{2}}}\). Compare the geometry around the silicon atoms in \({\text{Si}}{{\text{O}}_{\text{2}}}\) with the geometry around the xenon atoms in \({\text{Xe}}{{\text{O}}_{\text{2}}}\), using the valence shell electron pair repulsion (VSEPR) theory.
Antimony, Sb, forms a fluoride, \({\text{Sb}}{{\text{F}}_{\text{5}}}\).
The equilibrium that occurs when antimony(V) fluoride is dissolved in liquid hydrogen fluoride can be represented by the equation below.
\[{\text{Sb}}{{\text{F}}_5}{\text{(s)}} + {\text{2HF(l)}} \rightleftharpoons {\text{SbF}}_6^ - {\text{(sol)}} + {{\text{H}}_2}{{\text{F}}^ + }{\text{(sol)}}\]
Outline how the following factors account for the fact that HCl is a strong acid and HF is a weak acid.
Some students were provided with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a monobasic acid, HQ, and given the problem of determining whether HQ was a weak acid or a strong acid.
The second problem set for the students was to determine the acid dissociation constant, \({K_{\text{a}}}\), of the acid HQ and its \({\text{p}}{K_{\text{a}}}\).
State the element that you would expect to have chemical properties most similar to those of antimony.
Describe the relationship between \({\text{Sb}}{{\text{F}}_{\text{5}}}\) and \({\text{SbF}}_6^ - \) in terms of the Lewis theory of acids.
Explain the behaviour of HF in terms of the Brønsted–Lowry theory of acids.
The strength of the hydrogen–halogen bond.
The interaction between an undissociated hydrogen halide molecule and a water molecule.
Neelu and Charles decided to solve the problem by determining the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution needed to neutralize \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of the acid. Outline whether this was a good choice.
Identify one indicator that could be used when titrating aqueous sodium hydroxide with both a strong acid and a weak acid, and outline the reason for your choice.
Indicator:
Reason:
Neelu and Charles decided to compare the volume of sodium hydroxide solution needed with those required by known \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) strong and weak acids. Unfortunately they chose sulfuric acid as the strong acid. Outline why this was an unsuitable choice.
Francisco and Shamiso decided to measure the pH of the initial solution, HQ, and they found that its pH was 3.7. Deduce, giving a reason, the strength (weak or strong) of the acid HQ.
Explain how the \({\text{p}}{K_{\text{a}}}\) could be determined from a graph of pH against the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide added.
Francisco and Shamiso found that the pH of the initial \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution was 3.7. However, this reading was inaccurate because they forgot to wash the pH probe. Calculate the \({\text{p}}{K_{\text{a}}}\) of HQ using the reading they obtained.
The element boron has two naturally occurring isotopes, \(^{{\text{10}}}{\text{B}}\) and \(^{{\text{11}}}{\text{B}}\).
Phosphorus forms two chlorides, \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\).
Apply the Aufbau principle to state the full electron configuration for an atom of phosphorus.
Deduce the Lewis structures for \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\).
\({\text{PC}}{{\text{l}}_{\text{3}}}\)\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)\({\text{PC}}{{\text{l}}_{\text{5}}}\)
Predict the shapes and the bond angles in the two molecules.

Identify the type of hybridization present in \({\text{PC}}{{\text{l}}_{\text{3}}}\).
Compare the melting points of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\) and explain the difference.
Define an acid according to the Lewis theory.
State and explain the acid–base character of \({\text{PC}}{{\text{l}}_{\text{3}}}\) according to the Lewis theory.
Explain the delocalization of \(\pi \) electrons using the \({{\text{O}}_{\text{3}}}\) molecule as an example, including two facts that support the delocalization.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:

After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:

The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:
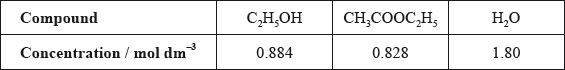
Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Ethanol has many industrial uses.
State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Students can also measure the enthalpy of combustion of ethanol in the laboratory using calorimetry. Suggest the major source of systematic error in these procedures.
State the equation for the acid-catalysed reaction of ethanol with propanoic acid and state the name of the organic product.
Equation:
Name of the organic product:
A polyester can be formed when ethane-1,2-diol reacts with benzene-1,4-dicarboxylic acid.
Deduce the structure of the repeating unit and state the other product formed.
Repeating unit:
Other product:
State the type of polymerization that occurs.
The standard enthalpy change of combustion, \(\Delta H_{\text{c}}^\Theta \), of propanoic acid is \( - 1527{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine the standard enthalpy change of formation of propanoic acid, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using this information and data from table 12 of the data booklet.
Deduce, giving a reason, the sign of the standard entropy change of the system for the formation of propanoic acid from its elements.
Identify three allotropes of carbon and describe their structures.
Two hydrides of nitrogen are ammonia and hydrazine, \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\). One derivative of ammonia is methanamine whose molecular structure is shown below.

Hydrazine is used to remove oxygen from water used to generate steam or hot water.
\[{{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}} + {{\text{O}}_{\text{2}}}{\text{(aq)}} \to {{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
The concentration of dissolved oxygen in a sample of water is \(8.0 \times {10^{ - 3}}{\text{ g}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
Ammonia reacts reversibly with water.
\[{\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{NH}}_{\text{4}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Explain the effect of adding \({{\text{H}}^ + }{\text{(aq)}}\) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
\[{{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {{\text{N}}_{\text{2}}}{\text{H}}_{\text{5}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
\[{\text{p}}{K_{\text{b}}}{\text{ (hydrazine)}} = 5.77\]
Calculate the pH of a \(0.0100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrazine.
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Determine the enthalpy change of reaction, \(\Delta H\), in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
\[{{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} \to {{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}}\]
The standard enthalpy of formation of \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(l)}}\) is \( + 50.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the enthalpy of vaporization, \(\Delta {H_{{\text{vap}}}}\), of hydrazine in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). \[{{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(l)}} \to {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}}\] (If you did not get an answer to (f), use \( - 85{\text{ kJ}}\) but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from \({\text{1000 d}}{{\text{m}}^{\text{3}}}\) of the sample.
Calculate the volume, in \({\text{d}}{{\text{m}}^{\text{3}}}\), of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = \({\text{24.8 d}}{{\text{m}}^{\text{3}}}\) at SATP.)
An organic compound, X, with a molar mass of approximately \({\text{88 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) contains 54.5% carbon, 36.3% oxygen and 9.2% hydrogen by mass.
Predict and explain the bond lengths and bond strengths of the carbon-oxygen bonds in \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}{{\text{O}}^ - }\).
(i) State the meaning of the term hybridization.
(ii) Describe the hybridization of the carbon atom in methane and explain how the concept of hybridization can be used to explain the shape of the methane molecule.
(iii) Identify the hybridization of the carbon atoms in diamond and graphite and explain why graphite is an electrical conductor.
Aluminium chloride, \({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\), does not conduct electricity when molten but aluminium oxide, \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\), does. Explain this in terms of the structure and bonding of the two compounds.
\({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\):
\({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
In December 2010, researchers in Sweden announced the synthesis of N,N–dinitronitramide, \({\text{N(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{3}}}\). They speculated that this compound, more commonly called trinitramide, may have significant potential as an environmentally friendly rocket fuel oxidant.
Methanol reacts with trinitramide to form nitrogen, carbon dioxide and water. Deduce the coefficients required to balance the equation for this reaction.
___ \({\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \) ___ \({\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \) ___ \({{\text{N}}_2}{\text{(g)}} + \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}} + \) ___ \({{\text{H}}_2}{\text{O(l)}}\)
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), when one mole of trinitramide decomposes to its elements, using bond enthalpy data from Table 10 of the Data Booklet. Assume that all the N–O bonds in this molecule have a bond enthalpy of \({\text{305 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The entropy change, \(\Delta S\), for the decomposition of trinitramide has been estimated as \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\). Comment on the sign of \(\Delta S\).
Using \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) as the value for the entropy change, along with your answer to part (c), calculate \(\Delta G\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for this reaction at 300 K. (If you did not obtain an answer for part (c), then use the value \( - 1000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Explain how changing the temperature will affect whether or not the decomposition of trinitramide is spontaneous.
Outline how the length of the N–N bond in trinitramide compares with the N–N bond in nitrogen gas, \({{\text{N}}_{\text{2}}}\).
Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
Predict, with an explanation, the polarity of the trinitramide molecule.
\({\text{B}}{{\text{F}}_{\text{3}}}{\text{(g)}}\) reacts with \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) to form \({{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) according to the equation below.
\[{\text{B}}{{\text{F}}_3}{\text{(g)}} + {\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}} \to {{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\]
The following is a proposed mechanism for the reaction of NO(g) with \({{\text{H}}_{\text{2}}}{\text{(g)}}\).
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{\text{2NO(g)}} \to {{\text{N}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}} \\ {{\text{Step 2:}}}&{{{\text{N}}_2}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \to {{\text{N}}_2}{\text{O(g)}} + {{\text{H}}_2}{\text{O(g)}}} \end{array}\]
Identify the type of bond present between \({\text{B}}{{\text{F}}_{\text{3}}}\) and \({\text{N}}{{\text{H}}_{\text{3}}}\) in \({{\text{F}}_{\text{3}}}{\text{BN}}{{\text{H}}_{\text{3}}}{\text{(g)}}\) and state another example of a compound with this type of bonding.
The table below shows initial rates of reaction for different concentrations of each reactant for this reaction at temperature, \(T\).

Deduce the rate expression, the overall order of the reaction and determine the value of \(k\), the rate constant, with its units, using the data from Experiment 4.
Identify the intermediate in the reaction.
The observed rate expression is \({\text{rate}} = k{{\text{[NO]}}^2}{\text{[}}{{\text{H}}_2}{\text{]}}\). Assuming that the proposed mechanism is correct, comment on the relative speeds of the two steps.
The following two-step mechanism has been suggested for the reaction of \({\text{N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) with CO (g), where \({k_2} \gg {k_1}\).
\[\begin{array}{*{20}{l}} {{\text{Step 1}}}&{{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{N}}{{\text{O}}_2}{\text{(g)}}\xrightarrow{{{k_1}}}{\text{NO(g)}} + {\text{N}}{{\text{O}}_3}{\text{(g)}}} \\ {{\text{Step 2:}}}&{{\text{N}}{{\text{O}}_3}{\text{(g)}} + {\text{CO(g)}}\xrightarrow{{{k_2}}}{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}} \\ {{\text{Overall:}}}&{{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}}\xrightarrow{{}}{\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}} \end{array}\]
The experimental rate expression is \({\text{rate}} = k{{\text{[N}}{{\text{O}}_2}{\text{]}}^2}\). Explain why this mechanism produces a rate expression consistent with the experimentally observed one.
HI(g) decomposes into \({{\text{H}}_2}{\text{(g)}}\) and \({{\text{I}}_{\text{2}}}{\text{(g)}}\) according to the reaction below.
\[{\text{2HI(g)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}}\]
The reaction was carried out at different temperatures and a value of the rate constant, \(k\), was obtained for each temperature. A graph of \(\ln k\) against \(\frac{1}{T}\) is shown below.
\(\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\)

Calculate the activation energy, \({E_{\text{a}}}\), for the reaction using these data and Table 1 of the Data Booklet showing your working.
Carboplatin used in the treatment of lung cancer has the following three-dimensional structure.

Elemental platinum has electrons occupying s, p, d and f atomic orbitals.
Identify the name of the functional group circled in the structure of carboplatin.
State the type of bonding between platinum and nitrogen in carboplatin.
Draw the shape of an s orbital and a px orbital. Label the x, y and z axes on each diagram.

State the maximum number of orbitals in the \(n = 4\) energy level.
A number of ruthenium-based anti-cancer drugs have also been developed. State the full electron configuration of the ruthenium(II) ion, \({\text{R}}{{\text{u}}^{2 + }}\).
Iron is in the same group in the periodic table as ruthenium.
Construct the orbital diagram (using the arrow-in-box notation) for iron, showing the electrons in the \(n = 3\) and \(n = 4\) energy levels only and label each sub-level on the diagram.

Graphite has a layered structure of carbon atoms. A section of the structure is shown below.

Identify the type of attraction represented by the dotted lines shown between the layers.
Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its layered structure.
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
A graph of the successive ionization energies of magnesium is shown below.

The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
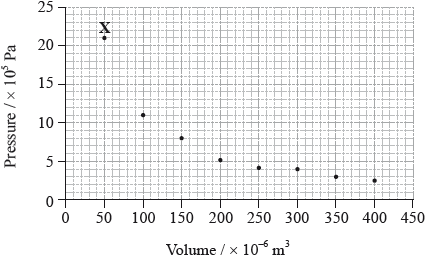
(i) Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
(ii) Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
(i) Explain the increase in ionization energy values from the 3rd to the 8th electrons.
(ii) Explain the sharp increase in ionization energy values between the 10th and 11th electrons.
(i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how the ions are formed, and the structure and bonding in magnesium oxide.
(ii) Carbon reacts with oxygen to form a covalent compound, carbon dioxide. Describe what is meant by a covalent bond.
(iii) State why magnesium and oxygen form an ionic compound while carbon and oxygen form a covalent compound.
(i) Predict the type of hybridization of the carbon and oxygen atoms in \({\text{C}}{{\text{O}}_{\text{2}}}\).
(ii) Sketch the orbitals of an oxygen atom in \({\text{C}}{{\text{O}}_{\text{2}}}\) on the energy level diagram provided, including the electrons that occupy each orbital.

(iii) Define the term electronegativity.
(iv) Explain why oxygen has a larger electronegativity than carbon.
(i) Draw a best-fit curve for the data on the graph.
(ii) Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.
(i) Most indicators are weak acids. Describe qualitatively how indicators work.
(ii) Identify a suitable indicator for a titration between a weak acid and a strong base, using Table 16 of the Data Booklet.
Some reactions of but-2-ene are given below.

But-2-ene can exist as two geometrical isomers. Cis-trans is a form of stereoisomerism.
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed by reacting compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), with aqueous potassium hydroxide. This reaction proceeds by both \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms. Explain the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism, using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C.
Deduce the equation for the complete combustion of compound C.
Define the term stereoisomers.
State the conditions needed for a compound to show cis-trans.
Draw the structures of the two geometrical isomers of but-2-ene, clearly identifying each as cis or trans.
Phosphoryl chloride, \({\text{POC}}{{\text{l}}_{\text{3}}}\), is a dehydrating agent.
\({\text{POC}}{{\text{l}}_{\text{3}}}\left( {\text{g}} \right)\) decomposes according to the following equation.
\[{\text{2POC}}{{\text{l}}_3}{\text{(g)}} \to {\text{2PC}}{{\text{l}}_3}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}}\]
POCl3 can be prepared by the reaction of phosphorus pentachloride, PCl5 , with tetraphosphorus decaoxide, P4O10.
PCl3 and Cl– can act as ligands in transition metal complexes such as Ni(PCl3)4 and [Cr(H2O)3Cl3].
Predict and explain the sign of the entropy change, \(\Delta S\), for this reaction.
Calculate the standard entropy change for the reaction, \(\Delta {S^\Theta }\), in \({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.

Define the term standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \).
Calculate the standard enthalpy change for the reaction, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.

Determine the standard free energy change for the reaction, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K.
Deduce the temperature, in K, at which the reaction becomes spontaneous.
Deduce the Lewis (electron dot) structure of POCl3 (with P as the central element) and PCl3 and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
State and explain the Cl–P–Cl bond angle in PCl3.
Deduce the Lewis (electron dot) structure of PCl5.
Predict the shape of this molecule, using the valence shell electron pair repulsion theory (VSEPR).
Identify all the different bond angles in PCl5.
PCl3Br2 has the same molecular shape as PCl5. Draw the three isomers of PCl3Br2 and deduce whether each isomer is polar or non-polar.
Define the term ligand.
Explain why the complex [Cr(H2O)3Cl3] is coloured.
Consider the structure and bonding in \({\text{MgC}}{{\text{l}}_{\text{2}}}\) and \({\text{PC}}{{\text{l}}_{\text{3}}}\).
Consider the molecules \({\text{PB}}{{\text{r}}_{\text{3}}}\) and \({\text{S}}{{\text{F}}_{\text{4}}}\).
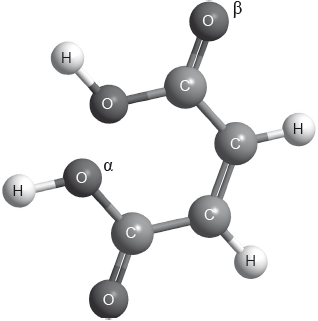
The structure of cis-but-2-ene-1,4-dioic acid is shown below.
State and explain the electrical conductivities of these two chloride compounds in their liquid state.
Suggest, giving your reasons, the approximate pH values of the solutions formed by adding each chloride compound separately to distilled water.
\({\text{MgC}}{{\text{l}}_{\text{2}}}\)
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
Identify the acid-base character of the oxides of each of the elements from sodium to chlorine in period 3.
State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with water.
Deduce the Lewis (electron dot) structure of both molecules.
Predict the shapes of the two molecules, giving the Br–P–Br bond angle in \({\text{PB}}{{\text{r}}_{\text{3}}}\) and the F–S–F bond angles in \({\text{S}}{{\text{F}}_{\text{4}}}\).

Explain why both \({\text{PB}}{{\text{r}}_{\text{3}}}\) and \({\text{S}}{{\text{F}}_{\text{4}}}\) are polar.
Describe the covalent bond between carbon and hydrogen in the molecule above and how it is formed.
Deduce the hybridization of the oxygen atoms labelled \(\alpha \) and \(\beta \).
\(\alpha \):
\(\beta \):
Describe sigma \((\sigma )\) and pi \((\pi )\) bonds between atoms.
\(\sigma \) bond:
\(\pi \) bond:
Identify the number of sigma \((\sigma )\) and pi \((\pi )\) bonds present in a molecule of cis-but-2-ene-1,4-dioic acid.
The oxides and chlorides of period 3 elements exhibit periodicity.
Chlorine gas, \({\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\), is bubbled through separate solutions of aqueous bromine, \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\), and potassium bromide, \({\text{KBr(aq)}}\).
The hydrogen halides do not show perfect periodicity. A bar chart of boiling points shows that the boiling point of hydrogen fluoride, HF, is much higher than periodic trends would indicate.

Transition metals form complex ions which are usually coloured.
(i) State the changes in the acid-base nature of the oxides across period 3 (from \({\text{N}}{{\text{a}}_2}{\text{O}}\) to \({\text{C}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{7}}}\)), including equations for the reactions of \({\text{N}}{{\text{a}}_2}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) with water.
(ii) State whether or not molten aluminium chloride, \({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\), and molten aluminium oxide, \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\), conduct electricity. Explain this behaviour in terms of the structure and bonding of the two compounds.
(iii) State the equation for the reaction of \({\text{C}}{{\text{l}}_{\text{2}}}\) with water.
(i) Predict any changes that may be observed in each case.
\({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\):
\({\text{KBr(aq)}}\):
(ii) State the half-equations for the reactions that occur.
(i) Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
(ii) Explain the trend in the boiling points of HCl, HBr and HI.
State the full electron configurations of Cr and \({\text{C}}{{\text{r}}^{3 + }}\).
Cr:
\({\text{C}}{{\text{r}}^{3 + }}\):
\({\text{C}}{{\text{r}}^{3 + }}\) ions and water molecules bond together to form the complex ion \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\).
Describe how the water acts and how it forms the bond, identifying the acid-base character of the reaction.
Explain why the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is coloured.
Outline, including a relevant equation, whether the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is acidic, basic or neutral.
Explain how the number of electrons in the outer main energy level of phosphorus, P, can be determined using the data of successive ionization energies.
This question is about the compounds of some period 3 elements.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in terms of their bonding and structure.
Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and molten states. Complete the boxes with “yes” or “no”.

Predict and explain the pH of the following aqueous solutions, using equations to support your answer.
Ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\):
Sodium methanoate, \({\text{HCOONa(aq)}}\):
Consider the structure and bonding in \({\text{MgC}}{{\text{l}}_{\text{2}}}\) and \({\text{PC}}{{\text{l}}_{\text{5}}}\).
For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and \({\text{S}}{{\text{F}}_{\text{6}}}\):
State and explain the difference in the electrical conductivity in the liquid state of the two chlorides.
(i) deduce the Lewis structure.
(ii) predict the shape and bond angle.
(iii) predict and explain the molecular polarity.

Compare the formation of sigma (\(\sigma \)) and pi (\(\pi \)) bonds between the carbon atoms in a molecule of ethyne.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use.
In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl(aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
The standard electrode potential for the reduction of the chlorate(V) ion to the chloride ion is \( + 1.49{\text{ V}}\).
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium in aqueous chlorine, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Partial neutralization of chloric(I) acid creates a buffer solution. Given that the \({\text{p}}{K_{\text{a}}}\) of chloric(I) acid is 7.53, determine the pH of a solution that has \({\text{[HOCl]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[Cl}}{{\text{O}}^ - }{\text{]}} = 0.0500{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Describe, using HIn to represent the indicator in its acid form, why an indicator changes colour when excess alkali is added.
(i) Deduce a balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide from the appropriate half-equations.
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the final equation.

(i) Define the term standard electrode potential.
(ii) Referring to Table 14 of the Data Booklet, deduce, giving a reason, whether the oxidation of the chromium(III) ion to the dichromate(VI) ion by the chlorate(V) ion is energetically feasible.
2-methylbutan-2-ol, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C(OH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is a liquid with a smell of camphor that was formerly used as a sedative. One way of producing it starts with 2-methylbut-2-ene.
As well as 2-methylbutan-2-ol, the reaction also produces a small quantity of an optically active isomer, X.
2-methylbutan-2-ol can also be produced by the hydrolysis of 2-chloro-2-methylbutane, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CCl}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\), with aqueous sodium hydroxide.
2-chloro-2-methylbutane contains some molecules with a molar mass of approximately \({\text{106 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and some with a molar mass of approximately \({\text{108 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
2-chloro-2-methylbutane can also be converted into compound Z by a two-stage reaction via compound Y:

State the other substances required to convert 2-methylbut-2-ene to 2-methylbutan-2-ol.
Explain whether you would expect 2-methylbutan-2-ol to react with acidified potassium dichromate(VI).
State what is meant by optical activity.
State what optical activity indicates about the structure of the molecule.
Optical activity can be detected using a polarimeter. Explain how this works.
Deduce the structural formula of X.
Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
State the rate expression for this reaction and the units of the rate constant.
Suggest why, for some other halogenoalkanes, this hydrolysis is much more effective in alkaline rather than in neutral conditions.
Outline why there are molecules with different molar masses.
Draw the structure of Y.
State the reagent and any catalyst required for both the formation of Y and the conversion of Y into Z.
Formation of Y:
Conversion of Y into Z:
Nitrogen and silicon belong to different groups in the periodic table.
Draw the Lewis structures, state the shapes and predict the bond angles for the following species.
Consider the molecule \({\text{HCON}}{{\text{H}}_{\text{2}}}\).
Distinguish in terms of electronic structure, between the terms group and period.
State the maximum number of orbitals in the \(n = 2\) energy level.
\({\text{SiF}}_6^{2 - }\)
\({\text{NO}}_2^ + \)
Explain, using diagrams, why \({\text{N}}{{\text{O}}_{\text{2}}}\) is a polar molecule but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
Explain the term hybridization.
Describe how \(\sigma \) and \(\pi \) bonds form.
State the type of hybridization of the carbon and nitrogen atoms in \({\text{HCON}}{{\text{H}}_{\text{2}}}\).
Ozone, \({{\text{O}}_{\text{3}}}\), in the upper atmosphere prevents harmful UV radiation reaching the surface of the Earth.
State the shape of the ozone molecule and estimate the bond angle.
Shape:
Bond angle:
State the hybridization of the central oxygen atom.
In terms of \(\sigma \) and \(\pi \) bonds, describe the two oxygen-oxygen bonds in the Lewis structure.
The two oxygen-oxygen bonds in ozone are in fact of equal length. Deduce why this is the case and how the length of these would compare to oxygen-oxygen bond lengths in hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), and in the oxygen molecule, \({{\text{O}}_{\text{2}}}\).
Hydrazine, N2H4, is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{l)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(l)}}\]
The reaction between \({{\text{N}}_2}{{\text{H}}_4}({\text{aq)}}\) and \({\text{HCl(aq)}}\) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw the Lewis (electron dot) structure for N2H4 showing all valence electrons.
(ii) State and explain the H–N–H bond angle in hydrazine.
Hydrazine and ethene, C2H4, are hydrides of adjacent elements in the periodic table. The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
(i) The enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of liquid hydrazine is \({\text{50.6 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Use this value, together with data from Table 12 of the Data Booklet, to calculate the enthalpy change for this reaction.
(ii) Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
(iii) Identify the calculation that produces the most accurate value for the enthalpy change for the reaction given and explain your choice.
(iv) Calculate \(\Delta {S^\Theta }\) for the reaction using the data below and comment on its magnitude.

(v) Calculate \(\Delta {G^\Theta }\) for the reaction at 298 K.
(vi) Predict, giving a reason, the spontaneity of the reaction above at both high and low temperatures.
The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and HCl(aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
(iii) Suggest the type of hybridization shown by the nitrogen atoms in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
Carbon and silicon belong to the same group of the periodic table.
Describe the delocalization of pi (\(\pi \)) electrons and explain how this can account for the structure and stability of the carbonate ion, \({\text{CO}}_3^{2 - }\).
Explain the meaning of the term hybridization. State the type of hybridization shown by the carbon atoms in carbon dioxide, diamond, graphite and the carbonate ion.
Explain the electrical conductivity of molten sodium oxide and liquid sulfur trioxide.
Samples of sodium oxide and solid sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and predict the electrical conductivity of each of the solutions formed.
Bromine is a member of group 7, the halogens.
Iron is a transition metal.
Freshly prepared iron(II) bromide can be electrolysed both in the liquid state and in aqueous solution.
Explain the trend in reactivity of the halogens.
Deduce, using equations where appropriate, if bromine reacts with sodium chloride solution and with sodium iodide solution.
Describe the bonding in metals and explain their malleability.
List three characteristic properties of transition elements.
Identify the type of bonding between iron and cyanide in \({{\text{[Fe(CN}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 - }}\).
Deduce the oxidation number of iron in \({{\text{[Fe(CN}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 - }}\).
Draw the abbreviated orbital diagram for an iron atom using the arrow-in-box notation to represent electrons.
Draw the abbreviated orbital diagram for the iron ion in [Fe(CN)6]3– using the arrow-in-box notation to represent electrons.
Describe, using a diagram, the essential components of an electrolytic cell.
Describe the two ways in which current is conducted in an electrolytic cell.
Predict and explain the products of electrolysis of a dilute iron(II) bromide solution.
Identify another product that is formed if the solution of iron(II) bromide is concentrated.
Explain why this other product is formed.
Copper is a metal that has been used by humans for thousands of years.
State the full electron configuration of \(^{{\text{65}}}{\text{Cu}}\).
State one difference in the physical properties of the isotopes \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) and explain why their chemical properties are the same.
Physical:
Chemical:
Describe the bonding in solid copper.
Titanium and vanadium are consecutive elements in the first transition metal series.
\({\text{TiC}}{{\text{l}}_{\text{4}}}\) reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:

Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the \(_{{\text{22}}}^{{\text{48}}}{\text{Ti}}\) atom.

State the full electron configuration of the \(_{{\text{22}}}^{{\text{48}}}{\text{T}}{{\text{i}}^{2 + }}\) ion.
Suggest why the melting point of vanadium is higher than that of titanium.
Sketch a graph of the first six successive ionization energies of vanadium on the axes provided.

Explain why an aluminium-titanium alloy is harder than pure aluminium.
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
Outline why transition metals form coloured compounds.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, \({\text{TiC}}{{\text{l}}_{\text{4}}}\), melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Suggest a suitable indicator for this titration. Use section 22 of the data booklet.
(ii) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(iii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iv) Determine the percentage purity of the hydrated ethanedioic acid sample.
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Explain how ethanedioate ions act as ligands.
Bonds can be formed in many ways.
Bonds can be formed in many ways.
The equilibrium for a mixture of NO2 and N2O4 gases is represented as:
2NO2(g) \( \rightleftharpoons \) N2O4(g)
At 100°C, the equilibrium constant, Kc, is 0.21.
Discuss the bonding in the resonance structures of ozone.
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and \(0.10{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
Some physical properties of molecular substances result from the different types of forces between their molecules.
Resonance structures exist when a molecule can be represented by more than one Lewis structure.
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.

Deduce, giving a reason, the more likely structure.
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
There are several structural isomers with the molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{Br}}\).
All the isomers react when warmed with a dilute aqueous solution of sodium hydroxide according to the equation below.
\[{{\text{C}}_5}{{\text{H}}_{11}}{\text{Br}} + {\text{NaOH}} \to {{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}} + {\text{NaBr}}\]
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
The reaction with 1-bromopentane proceeds by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
The reaction with 2-bromo-2-methylbutane proceeds by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
Explain why 1-bromopentane reacts by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism whereas 2-bromo-2-methylbutane reacts by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Explain whether the boiling point of 1-bromopentane will be higher, lower or the same as that of 2-bromo-2-methylbutane.
The product \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) formed from the reaction with 1-bromopentane is warmed with ethanoic acid in the presence of a few drops of concentrated sulfuric acid. State the name of the type of reaction taking place and the structural formula of the organic product.
Consider the following reactions.

State the IUPAC names of each of the compounds, D, E, F and G.
D:
E:
F:
G:
State the reagents and reaction conditions used to convert D to E and D to F directly.
Discuss the volatility of E compared to F.
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
The electron configuration of boron is \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}\). Draw the shape of an s orbital and a \({{\text{p}}_x}\) orbital on the axes below.

(ii) Cobalt is a transition metal. One common ion of cobalt is \({\text{C}}{{\text{o}}^{3 + }}\). Draw the orbital diagram (using the arrow-in-box notation) for the \({\text{C}}{{\text{o}}^{3 + }}\) ion.

(iii) State the other most common ion of cobalt.
(iv) Explain why the complex \({\text{[Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]C}}{{\text{l}}_{\text{3}}}\) is coloured.
Two chemistry students wished to determine the enthalpy of hydration of anhydrous magnesium sulfate. They measured the initial and the highest temperature reached when anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}{\text{(s)}}\), was dissolved in water. They presented their results in the table below.

The students repeated the experiment using 6.16 g of solid hydrated magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}} \bullet {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(s)}}\), and \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. They found the enthalpy change, \(\Delta {H_2}\) , to be \( + {\text{18 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The enthalpy of hydration of solid anhydrous magnesium sulfate is difficult to determine experimentally, but can be determined using the diagram below.

(i) Calculate the amount, in mol, of anhydrous magnesium sulfate.
(ii) Calculate the enthalpy change, \(\Delta {H_1}\), for anhydrous magnesium sulfate dissolving in water, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). State your answer to the correct number of significant figures.
(i) Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the hydration of solid anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}\).
(ii) The literature value for the enthalpy of hydration of anhydrous magnesium sulfate is \( - 103{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage difference between the literature value and the value determined from experimental results, giving your answer to one decimal place. (If you did not obtain an answer for the experimental value in (b)(i) then use the value of \( - 100{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Another group of students experimentally determined an enthalpy of hydration of \( - 95{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Outline two reasons which may explain the variation between the experimental and literature values.
Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic limestone. This limestone is a mixture of magnesium carbonate and calcium carbonate.
(i) State the equation for the reaction of sulfuric acid with magnesium carbonate.
(ii) Deduce the Lewis (electron dot) structure of the carbonate ion, giving the shape and the oxygen-carbon-oxygen bond angle.
Lewis (electron dot) structure:
Shape:
Bond angle:
(iii) There are three possible Lewis structures that can be drawn for the carbonate ion, which lead to a resonance structure. Explain, with reference to the electrons, why all carbon-oxygen bonds have the same length.
(iv) Deduce the hybridization of the carbon atom in the carbonate ion.
Calcium nitrate contains both covalent and ionic bonds.
Nitrogen also forms oxides, which are atmospheric pollutants.
State the formula of both ions present and the nature of the force between these ions.
Ions:
Nature of force:
State which atoms are covalently bonded.
Bonding in the nitrate ion involves electron delocalization. Explain the meaning of electron delocalization and how it affects the ion.
Outline the source of these oxides.
State one product formed from their reaction with water.
State one environmental problem caused by these atmospheric pollutants.
Magnesium, a reactive metal found in many common minerals, is also an essential nutrient for both plants and animals.
Successive ionization energies of magnesium are given in the table below.

Magnesium metal is mainly used as a component in lightweight alloys, particularly in combination with aluminium and titanium.
Magnesium is usually produced by the electrolysis of molten magnesium chloride.
Define the term first ionization energy.
(i) Explain why the second ionization energy is greater than the first ionization energy.
(ii) Explain why the third ionization energy is much greater than the second ionization energy.
Although magnesium is usually found as \({\text{M}}{{\text{g}}^{2 + }}\) in its compounds, it is possible to use the Born-Haber cycle to investigate the possibility of \({\text{M}}{{\text{g}}^ + }\) being able to form stable compounds.
Use the ionization energy data from part (b), along with the other data provided below, to determine the enthalpy change of formation of MgCl(s). Assume that, because \({\text{M}}{{\text{g}}^ + }\) would be similar in size to \({\text{N}}{{\text{a}}^ + }\), MgCl would have a similar lattice enthalpy to NaCl.
Enthalpy of atomization of Mg \( + 146{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Bond enthalpy in \({\text{C}}{{\text{l}}_{\text{2}}}\) \( + 243{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Electron affinity of Cl \( + 349{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Lattice enthalpy of NaCl \( + 790{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Consider the lattice enthalpies of \({\text{Mg}}{{\text{F}}_{\text{2}}}\), \({\text{MgC}}{{\text{l}}_2}\) and \({\text{CaC}}{{\text{l}}_{\text{2}}}\). List these from the most endothermic to the least endothermic and explain your order.
\({\text{Most endothermic}} \to {\text{Least endothermic}}\)
Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in water and the equilibrium below exists when excess solid is in contact with a saturated solution.
\[{\text{Mg(OH}}{{\text{)}}_2}{\text{(s)}} \rightleftharpoons {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline how the solubility of magnesium hydroxide will vary with pH.
(i) Describe the bonding present in magnesium metal.
(ii) Suggest why magnesium is harder than sodium.
(iii) Outline why alloys are generally less malleable than their component metals.
(i) Draw a labelled diagram of a suitable apparatus for the electrolysis.
(ii) State equations for the reactions that take place at the electrodes.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iii) When dilute aqueous magnesium chloride is used as the electrolyte, the reactions at both electrodes are different. State equations for the reactions that occur in aqueous solution.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iv) Outline why magnesium metal is not produced in the electrolysis of aqueous magnesium chloride.
An equilibrium exists between nitrosyl chloride, NOCl, nitrogen oxide, NO, and chlorine, \({\text{C}}{{\text{l}}_{\text{2}}}\).
\[{\text{2NOCl(g)}} \rightleftharpoons {\text{2NO(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}}\]
\({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of hexane, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{14}}}}\), and \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of pentan-1-ol, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\), were placed separately into two closed containers at 298 K and allowed to reach equilibrium.
Ammonia is a weak base.
(i) Deduce the equilibrium constant expression for this reaction.
(ii) Explain the effect on the position of equilibrium and the value of \({K_{\text{c}}}\) when pressure is decreased and temperature is kept constant.
(iii) 2.00 mol of NOCl was placed in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container and allowed to reach equilibrium at 298 K. At equilibrium, 0.200 mol of NO was present. Determine the equilibrium concentrations of NOCl and \({\text{C}}{{\text{l}}_{\text{2}}}\), and hence calculate the value of \({K_{\text{c}}}\) at this temperature.
(iv) The value of \({K_{\text{c}}}\) is \(1.60 \times {10^{ - 5}}\) at 318 K. State and explain whether the forward reaction is exothermic or endothermic.
(i) Compare the two liquids in terms of their boiling points, enthalpies of vaporization and vapour pressures.
(ii) Explain your answer given for part (b)(i).
Calculate the pH of a \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia at 298 K to two decimal places, using Table 15 of the Data Booklet.
A buffer solution is made using \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid, HCl (aq), and \({\text{20.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ammonia solution, \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\).
Describe the meaning of the term buffer solution.
Determine the pH of the buffer solution at 298 K.
A \({\text{1.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia is added to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution in a titration experiment.
Calculate the total volume of the solution at the equivalence point.
Calculate the pH of the solution at the equivalence point, using Table 15 of the Data Booklet.
Identify a suitable indicator for this titration, using Table 16 of the Data Booklet.
Draw the Lewis structures, state the shape and predict the bond angles for the following species.
Consider the following Born-Haber cycle:

The magnitudes for each of the enthalpy changes (a to e) are given in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but their signs (+ or –) have been omitted.
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
\({\text{NH}}_2^ - \)
\({\text{Xe}}{{\text{F}}_{\text{4}}}\)
State the names for the enthalpy changes c and d.
Deduce which two of the enthalpy changes a to e have negative signs.
Determine the value for the enthalpy of formation of potassium bromide.
Explain why the quantitative value for the lattice enthalpy of calcium bromide is larger than the value for the lattice enthalpy of potassium bromide.
Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon atoms in a molecule.
Identify how many sigma and pi bonds are present in propene, \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\).
Deduce all the bond angles present in propene.
Explain how the concept of hybridization can be used to explain the bonding in the triple bond present in propyne.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.

(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
State the relationship between the electron arrangement of an element and its group and period in the periodic table.
Transition metals and their compounds often catalyse reactions. The catalyzed decomposition of hydrogen peroxide by CuO is an example. State two other examples of catalyzed reactions giving the transition metal or its compound acting as catalyst.
(i) State a chemical equation for the partial dissociation of water into ions, including state symbols.
(ii) The dissociation of water into ions is reversible. State the expression for the ionic product constant of water.
(iii) The ionic product constant of water was measured at three different temperatures.

Deduce whether the ionization of water is exothermic or endothermic, giving your reason.
(iv) Use the data in part (iii) to determine the pH of water at 373 K, correct to two decimal places.
(i) An aqueous solution of sodium chloride is electrolysed using inert electrodes. Explain which product is obtained at the positive electrode (anode) if the concentration of sodium chloride is high.
(ii) State the half-equations occurring at the electrodes during the electrolysis of the concentrated aqueous solution of sodium chloride.
Negative electrode (cathode):
Positive electrode (anode):
Describe how electrolysis can be used to electroplate a bracelet with a layer of silver metal. Include the choice of electrodes and electrolyte needed in your description.
EUK-134, the structure of which is shown below, is a complex ion of manganese(III) that is used in expensive sun-protection products because of its powerful antioxidant properties.

State the electron configuration of the manganese ion in EUK-134.
State the name given to species that bond to a central metal ion, and identify the type of bond present.
Name given:
Type of bond:
Transition metals have certain characteristic properties. State two properties that are involved in EUK-134 rapidly decreasing the concentration of oxidizing agents.
Substances like EUK-134 are often coloured. Explain why compounds of transition metals absorb visible radiation.
But-2-ene is a straight-chain alkene with formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\). The molecule contains both \(\sigma \) and \(\pi \) bonds.

The polymerization of the alkenes is one of the most significant reactions of the twentieth century.
(i) Explain the formation of the \(\pi \) bond.
(ii) For each of the carbon atoms, C(1) and C(2), identify the type of hybridization shown.
C(1):
C(2):
But-2-ene shows geometrical isomerism. Draw the structural formula and state the name of the other geometrical isomer.
Identify the structural formula of an isomer of but-2-ene which does not decolourize bromine water, Br2(aq).
(i) Outline two reasons why the polymers of the alkenes are of economic importance.
(ii) State the type of polymerization reaction shown by the alkene in part (a).
(iii) Deduce the structure of the resulting polymer showing three repeating units.
(iv) Explain why monomers are often gases or volatile liquids, but polymers are solids.
Chromium is a transition metal with many uses.
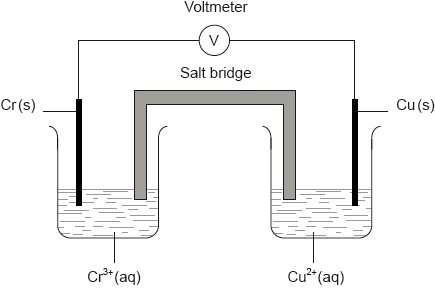
A voltaic cell is constructed as follows. One half-cell contains a chromium electrode immersed in a solution containing \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) ions. The other half-cell contains a copper electrode immersed in a solution containing \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) ions. The two electrodes are connected to a voltmeter and the two solutions by a salt bridge.
Draw an orbital diagram (using the arrow-in-box notation) showing the electrons in the 4s and 3d sub-levels in chromium metal.
Outline the nature of the metallic bonding present in chromium.
Explain why chromium metal is malleable.
State the name of \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\).
Describe the ionic bonding present in \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) and how the ions are formed.
Suggest why solid \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) does not conduct electricity.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Deduce the oxidation number of chromium in this complex.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Describe the nature of the ligand-chromium ion bonds in terms of acid-base theory.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Explain why \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{]}}^ + }\) is coloured.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Draw the structures of two possible isomers of this complex ion.
The dichromate ion, \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }{\text{(aq)}}\), and the iodide ion, \({{\text{I}}^ - }{\text{(aq)}}\), react together in the presence of an acid to form \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) and \({\text{IO}}_3^ - {\text{(aq)}}\) ions. Deduce the half-equation for the reaction of \({{\text{I}}^ - }\) to \({\text{IO}}_3^ - \) and the overall equation for this reaction.
Half-equation:
Overall equation:
Explain in terms of oxidation numbers whether iodine is oxidized or reduced in part (d) (i).
Define the term standard electrode potential.
Calculate the cell potential, in V, under standard conditions, for this voltaic cell, using table 14 of the data booklet and \({\text{E}}_{{\text{C}}{{\text{r}}^{3 + }}/{\text{Cr}}}^\Theta = -0.74{\text{ V}}\).
Predict the balanced equation for the spontaneous reaction which will produce a current in this voltaic cell.
Identify the negative and the positive electrodes in this cell.
Predict the direction of movement of electrons in the external circuit.
State the directions in which the negative ions (anions) and the positive ions (cations) flow in the salt bridge.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi (\(\pi \)) bonds are formed.
Deduce the number of σ and \(\pi \) bonds in a molecule of ethyne.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
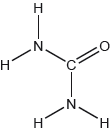
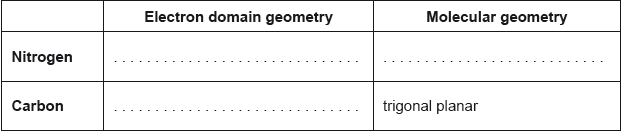
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
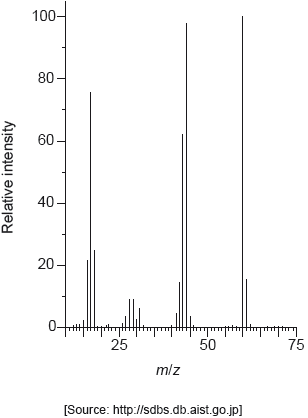
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.

Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Trends in physical and chemical properties are useful to chemists.
Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.
Describe, in terms of acid-base theories, the type of reaction that takes place between the cobalt ion and water to form the complex ion.
Phosphine (IUPAC name phosphane) is a hydride of phosphorus, with the formula PH3.
(i) Draw a Lewis (electron dot) structure of phosphine.
(ii) State the hybridization of the phosphorus atom in phosphine.
(iii) Deduce, giving your reason, whether phosphine would act as a Lewis acid, a Lewis base, or neither.
(iv) Outline whether you expect the bonds in phosphine to be polar or non-polar, giving a brief reason.
(v) Phosphine has a much greater molar mass than ammonia. Explain why phosphine has a significantly lower boiling point than ammonia.
(vi) Ammonia acts as a weak Brønsted–Lowry base when dissolved in water.
Outline what is meant by the terms “weak” and “Brønsted–Lowry base”.
Weak:
Brønsted–Lowry base:
Phosphine is usually prepared by heating white phosphorus, one of the allotropes of phosphorus, with concentrated aqueous sodium hydroxide. The equation for the reaction is:
(i) The first reagent is written as P4, not 4P. Describe the difference between P4 and 4P.
(ii) The ion H2PO2− is amphiprotic. Outline what is meant by amphiprotic, giving the formulas of both species it is converted to when it behaves in this manner.
(iii) State the oxidation state of phosphorus in P4 and H2PO2−.
P4:
H2PO2−:
(iv) Oxidation is now defined in terms of change of oxidation number. Explore how earlier definitions of oxidation and reduction may have led to conflicting answers for the conversion of P4 to H2PO2− and the way in which the use of oxidation numbers has resolved this.
2.478 g of white phosphorus was used to make phosphine according to the equation:
(i) Calculate the amount, in mol, of white phosphorus used.
(ii) This phosphorus was reacted with 100.0 cm3 of 5.00 mol dm−3 aqueous sodium hydroxide. Deduce, showing your working, which was the limiting reagent.
(iii) Determine the excess amount, in mol, of the other reagent.
(iv) Determine the volume of phosphine, measured in cm3 at standard temperature and pressure, that was produced.
Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and water.
(i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol phosphine. Calculate the temperature rise using section 1 of the data booklet and the data below.
Standard enthalpy of combustion of phosphine,
Specific heat capacity of air = 1.00Jg−1K−1=1.00kJkg−1K−1
(ii) The oxide formed in the reaction with air contains 43.6% phosphorus by mass. Determine the empirical formula of the oxide, showing your method.
(iii) The molar mass of the oxide is approximately 285 g mol−1. Determine the molecular formula of the oxide.
(iv) State the equation for the reaction of this oxide of phosphorus with water.
(v) Suggest why oxides of phosphorus are not major contributors to acid deposition.
(vi) The levels of sulfur dioxide, a major contributor to acid deposition, can be minimized by either pre-combustion and post-combustion methods. Outline one technique of each method.
Pre-combustion:
Post-combustion:
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
Predict whether the molecules PF3 and PF5 are polar or non-polar.
State the type of hybridization shown by the phosphorus atom in PF3.
The reaction between hydrogen and nitrogen monoxide is thought to proceed by the mechanism shown below.
(i) State the equation for the overall reaction.
(ii) Deduce the rate expression consistent with this mechanism.
(iii) Explain how you would attempt to confirm this rate expression, giving the results you would expect.
(iv) State, giving your reason, whether confirmation of the rate expression would prove that the mechanism given is correct.
(v) Suggest how the rate of this reaction could be measured experimentally.
The enthalpy change for the reaction between nitrogen monoxide and hydrogen is −664 kJ and its activation energy is 63 kJ.
(i) Sketch the potential energy profile for the overall reaction, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.
One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen monoxide, N2O. This can be represented by the resonance structures below:
(i) Analyse the bonding in dinitrogen monoxide in terms of σ-bonds and Δ-bonds.
(ii) State what is meant by resonance.